|
HOME >>
API >>
Etafedrine

|
(l)-Etafedrine Hydrochloride

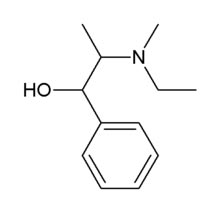 |
Molecular Formula : C12H19NO.HCl
Molecular Weight : 229.75
CAS No. : [5591-29-7]
Chemical Name :
1. (1R,2S)-2-[Ethyl (methyl)amino]-1-phenylpropan-1-ol
Hydrochloride.
2. (1R,2S)-2-Ethylmethylamino-1-phenyl-1-propanol Hydrochloride
Specifications : |
| 1. |
Description : |
White to off
white crystalline powder |
| 2. |
Identification: |
Infrared spectrum of sample
is concordant with standard. |
| 3. |
Melting range : |
183° - 186°C |
| 4. |
Specific rotation : |
- 17.5° - 19.5° (5 % solution
in water) |
| 5. |
Moisture content : |
Not more than 0.5 % w/w |
| 6. |
Sulphated ash : |
Not more than 0.1 % w/w |
| 7. |
Assay (Non.Aq.
Titration) : |
Between 99.0 and 100.5 % w/w
(dried basis) |
Therapeutic indications :
Bronchodilator
Packing
: Packed in double lined polyethylene bags in 10 kg
/ 25 kg fiber drums.
Storage
: Store below 35°C
IUPAC
Name: 2-[ethyl(methyl)amino]-1-phenylpropan-1-ol
hydrochloride
CAS Registry Number: 5591-29-7
Synonyms: Nethamine, Nethaphyl,
Etafedrine hydrochloride, Nethamine (TN), Etafedrine hydrochloride (USAN),
Etafedrine hydrochloride [USAN], CID68646, EINECS 227-000-5, D04072,
alpha-(1-(Ethylmethylamino)ethyl)benzyl alcohol hydrochloride,
Benzenemethanol, alpha-(1-(ethylmethylamino)ethyl)-, hydrochloride,
5591-29-7, BENZYL ALCOHOL, alpha-(1-(ETHYLMETHYLAMINO)ETHYL)-,
HYDROCHLORIDE, (-)-, 530-35-8, 7681-79-0
Molecular Formula: C12H20ClNO
Molecular Weight: 229.746300 [g/mol]
H-Bond Donor: 2 H-Bond Acceptor: 2
Etafedrine were studied on the tracheal chain preparation and
the atria of the guinea pig and on the rabbit perfused heart.
Contraction of the tracheal chain by acetylcholine or histamine was
antagonized by epinephrine (adrenaline), etafedrine and ephedrine,
the relative potencies being 91:1:0.3, respectively.
Propranolol (5 X 10(-7) mol/l) completely antagonized the
bronchodilator effect of 10(-4) mol/l etafedrine on the
acetylcholine-evoked contraction. Etafedrine up to 3 X 10(-4) mol/l
did not increase heart rate or force of contraction in guinea pig
atria. In contrast to tyramine and ephedrine (both 10(-5) mol/l),
etafedrine (10(-4) mol/l) failed to release 3H-norepinephrine (noradrenaline)
in the perfused rabbit heart. Moreover, the concentration-dependent
positive chronotropic and inotropic effects of norepinephrine in
isolated atria were slightly, but not significantly enhanced by
etafedrine.
It is concluded that N-ethylation of ephedrine suppresses the
indirect sympathomimetic activity and markedly enhances the efficacy
on beta 2-adrenoceptors.
Etafedrine appears to be a sympathomimetic agent with a selective
activity on beta 2-adrenoceptors.
PHARMACOLOGICAL ACTION:
Etafedrine a sympathomimetic agent, acts on the sympathetic
receptors of the bronchial tree relaxing spasm in a manner similar
to that of ephedrine.
It helps to control cough associated with inflammation of the mouth
and throat that is not helped by cough medications that are less
strong.
Hydrocodone is a narcotic medication that is an antitussive (cough
suppressant). It helps to reduce cough by affecting the cough centre
in the brain.
Etafedrine belongs to the family of medications called
decongestants. It works by narrowing blood vessels in the nasal
passages, helping to relieve nasal stuffiness.
Do not give this medication to anyone else, even if they have the
same symptoms as you do. It can be harmful for people to take this
medication if their doctor has not prescribed it.
The recommended dose of this medication is
Adults: 5 mL (1 teaspoonful) every 3 to 5 hours as needed, but not
more than 30 mL in any 24-hour period.
Children 6 to 12 years of age: 2.5 mL to 5 mL every 3 to 5 hours as
needed to a maximum of 15 mL in any 24-hour period.
Children 1 to 6 years of age: 1.25 mL to 2.5 mL every 3 to 5 hours
as needed to a maximum of 3 doses in any 24-hour period.
Use an oral syringe to measure each dose of the liquid as it gives a
more accurate measurement than household teaspoons.
Store this medication at room temperature, protect it from light,
and keep it out of the reach of children.
Do not dispose of medications in wastewater (e.g. down the sink or
in the toilet) or in household garbage. Ask your pharmacist how to
dispose of medications that are no longer needed or have expired.
Side effects occur
* fast, slow, or pounding heartbeat
* hallucinations (seeing, hearing, or feeling things that are not
there)
* hives, itching, or skin rash
* increased sweating
* irregular breathing
* mental depression or other mood or mental changes
* redness or flushing of face
* ringing or buzzing in the ears
* shortness of breath, wheezing, or troubled breathing
* swelling of face
* trembling or uncontrolled muscle movements
* unusual excitement or restlessness (especially in children)
Stop taking the medication and seek immediate medical attention
if any of the following occur:
* cold, clammy skin
* headache (severe or continuing)
* low blood pressure
* pinpoint pupils of eyes
* seizures
* severe confusion or disorientation
* severe dizziness
* severe drowsiness
* severe nausea or vomiting
* severe nervousness or restlessness
* severe weakness
* shortness of breath or troubled breathing (severe or continuing)
* signs of a severe allergic reaction such as hives; difficulty
breathing; swelling of the tongue, face, mouth, or throat
* slow heartbeat
Etafedrine (INN) or ethylephedrine is a long-acting
bronchodilator and has the brand name Nethaprin. It is
commercially available as both the free base, and as the
hydrochloride.
Ethylephedrine may be synthesized by alkylating ephedrine with ethyl
iodide. The hydrochloride may be prepared by passing hydrogen
chloride through a solution of ethylephedrine in diethyl ether.
 Note:
These API/ chemicals are designated as those that are used in
the manufacture of the controlled substances and are important to
the manufacture of the substances. For any (Control Substance)
products Import and Export *** subjected to your country government
laws /control substance ACT. Note:
These API/ chemicals are designated as those that are used in
the manufacture of the controlled substances and are important to
the manufacture of the substances. For any (Control Substance)
products Import and Export *** subjected to your country government
laws /control substance ACT.
Note /Government Notification:
These chemicals are designated as those that are used in the
manufacture of the controlled substances and are important to the
manufacture of the substances. For any (Control Substance) products
Import and Export *** subjected to your country government laws
/control substance ACT.
Information: The information on this web page is provided to
help you to work safely, but it is intended to be an overview of
hazards, not a replacement for a full Material Safety Data Sheet (MSDS).
MSDS forms can be downloaded from the web sites of many chemical
suppliers. ,also that the information on the PTCL Safety web site,
where this page was hosted, has been copied onto many other sites,
often without permission. If you have any doubts about the veracity
of the information that you are viewing, or have any queries, please
check the URL that your web browser displays for this page. If the
URL begins "www.tajapi.com/www/Denatonium Benzoate.htm/" the page is
maintained by the Safety Officer in Physical Chemistry at Oxford
University. If not, this page is a copy made by some other person
and we have no responsibility for it.
The Controlled Substances Act (CSA) was enacted into law by the
Congress of the United States as Title II of the Comprehensive Drug
Abuse Prevention and Control Act of 1970.[1] The CSA is the federal
U.S. drug policy under which the manufacture, importation,
possession, use and distribution of certain substances is regulated.
The Act also served as the national implementing legislation for the
Single Convention on Narcotic Drugs.
New Added Products:
Phenobarbital sodium [57-30-7],Acenocoumarol
[152-72-7],Esomeprazole
Magnesium,Famotidine,Ibuprofen,Lacidipine,Levetiracetam,Losartan
Potassium,
Montelukast
Sodium,Naproxen,Norfloxacin,Olmesartan,Omeprazole
Sodium,
Pantoprazole,Quetiapine
Fumarate,TrandolaprilZolmitriptan
|


|


![etafedrine Molecular Weight: 229.746300 [g/mol]](new%20update1/etafedrine_img1.jpg)











