|
|

Chemical Formulas
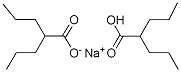
Divalproex
Sodium Uses
Pharmacodynamics Divalproex sodium dissociates to the valproate ion
in the gastrointestinal tract. The mechanisms by which valproate
exerts its therapeutic effects have not been established. It has
been suggested that its activity in epilepsy is related to increased
brain concentrations of gamma-aminobutyric acid.
Pharmacokinetics
However, it is possible that differences among the various valproate
products in Tmax and Cmax could be important upon initiation of
treatment. For example, in single dose studies, the effect of
feeding had a greater influence on the rate of absorption of the
tablet (increase in Tmax from 4 to 8 hours) than on the absorption
of the sprinkle capsules (increase in Tmax from 3.3 to 4.8 hours).
Divalproex sodium dissociates to the valproate ion in the
gastrointestinal tract. The mechanisms by which valproate exerts its
therapeutic effects have not been established. It has been suggested
that its activity in epilepsy is related to increased brain
concentrations of gamma-aminobutyric acid.
In five multiple-dose studies in healthy subjects (N= 82) and in
subjects with epilepsy (N= 86), when administered under fasting and
nonfasting conditions, given once daily produced an average
bioavailability of 89% relative to an equal total daily dose of
given BID, TID, or QID. The median time to maximum plasma valproate
concentrations (Cmax) after administration ranged from 4 to 17
hours. After multiple once-daily dosing of the peak-to-trough
fluctuation in plasma valproate concentrations was 10-20% lower than
that of regular.
A new oral polymeric controlled release formulation suitable for the
once-a-day administration of valproate compounds, such as divalproex
sodium, has been discovered. This formulation exhibits significant
advantages over the sustained release valproate formulations of the
prior art. This formulation minimizes the variation between peak and
trough plasma levels of valproate over a 24 hour dosing period. This
formulation follows a zero-order release pattern thus producing
essentially flat plasma levels of valproate, once steady-state
levels have been achieved. This results in a significantly lower
incidence of side effects for patients consuming such a formulation.
Information
Associated with Product :
Dosage
Uses
Side Effects
Pharmacology
>>


PDF DOWNLOAD
WORD DOCUMENT
|
|
|
|